I get asked this question a lot— “How much ammonia is in our quat?” Also, from water treatment companies or waste water facilities I hear, “When quat breaks down, how much ammonia is released?”
The answer to both is “none”. Alkyl quaternium compounds (quats) do not contain or release ammonia.
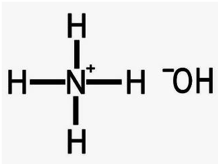
Ammonia is a “term” used for what we buy at the grocery store for cleaning windows. It is actually ammonium hydroxide. (NH4 OH) Ammonium hydroxide has a very low molecular weight of 34, which is, of course, volatile. The compound that is actually ammonia, is a nitrogen atom that is connected to 3 hydrogen atoms (NH3) and is a corrosive, alkaline, highly volatile gas. When diluted in water, ammonia becomes ammonium hydroxide, and that is really what is bottled for sale in the grocery stores.
Ammonia Hydroxide
Alkyl quaternium compounds (also referred to as alkyl quaternary ammonium compounds or quats), by contrast, are non-volatile compounds based on coconut oil. Once you add water to ammonia to form ammonia hydroxide, the nitrogen atom is connected to 4 hydrogen atoms (see above).
In alkyl quaternary ammonium compounds the nitrogen atom is connected to 1 or more coconut oil alkyl groups, (50% C14, 40% C12 & 10% C16 as in Maquat MC1412) and 2-3 methyl or methylbenzyl groups, with no hydrogen atoms connected to the nitrogen atom. The molecular weights of quats range from 300-400, and are completely non-volatile.
Typical ADBAC Quat
At no point in the manufacturing of alkyl quaternary ammonium compounds is ammonia or ammonium hydroxide an ingredient or potential degradation product.